Pontiuth Pilate
Republican Jesus!
I have strips of common metals (copper, nickel, iron, zinc and aluminum) as well as nitrate solutions of each of these. I want to build two batteries in series by connecting two galvanic cells with a third wire. Schematic:
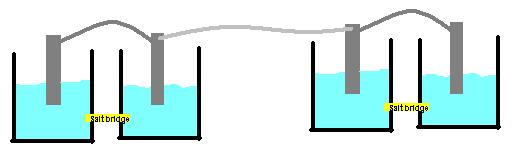
Some questions:
1. Do I need a third salt bridge between the cells?
2. Do I need to take into account the two reactions, or three including the one occuring across the wire between the two cells?
3. What metals should I use to achieve the maximum possible voltage? Will the voltages sum across the series or am I doing something wrong?
Some questions:
1. Do I need a third salt bridge between the cells?
2. Do I need to take into account the two reactions, or three including the one occuring across the wire between the two cells?
3. What metals should I use to achieve the maximum possible voltage? Will the voltages sum across the series or am I doing something wrong?
