Perfection
The Great Head.
Liquid carbon is a major pain to create, but it can be done take a look at the phase diagram to see why... http://en.wikipedia.org/wiki/Image:Carbon_basic_phase_diagram.pngThe Last Conformist said:Hm? If so, my chemistry textbooks lied to me.
Liquid CO2 is really easy to make, though. You just need a high pressure environment.
Edit: Whoops, it's high pressure, silly me.

 )
)
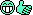 Now I can go to dinners and be a wise-ass
Now I can go to dinners and be a wise-ass 