ironduck
Deity
- Joined
- Oct 13, 2002
- Messages
- 6,561
Till said:How about an image contest of Particular Pretty Proteins to un-geek things?
Yeah, I was thinking about that too - similar to the astronomy picture thread.
Till said:How about an image contest of Particular Pretty Proteins to un-geek things?

 That's because it's off the web, rather than a F@H! Cheat! Cheat!
That's because it's off the web, rather than a F@H! Cheat! Cheat! 

Sophie 378 said:Shall I spam this thread with pretty protein pics?
I have lots - dissertation on metalloproteins, and lots of lectures and papers!









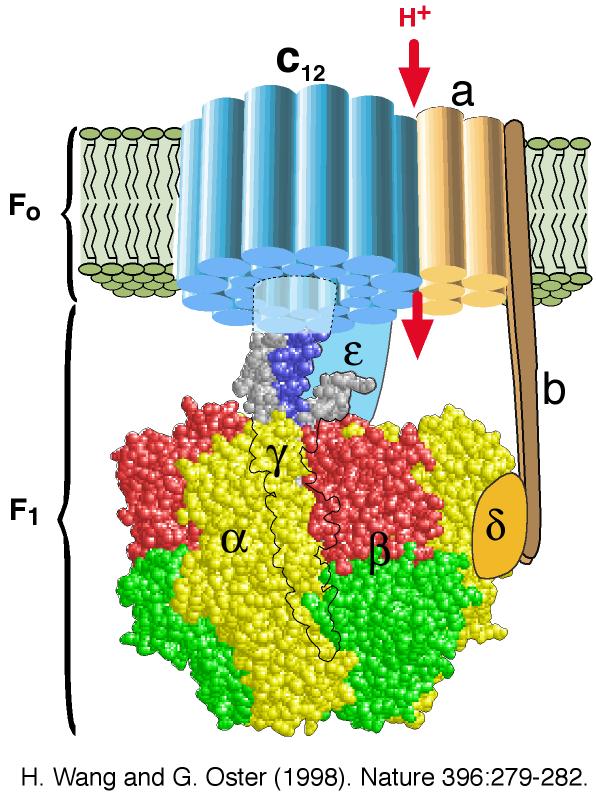

 - I was just of the understanding that there are high enough resolution images that shows individual proteins reasonably well..
- I was just of the understanding that there are high enough resolution images that shows individual proteins reasonably well..
I'm no good with organometallic compounds. What the heck kind of bonds are between the calcium and the other stuff?Sophie 378 said:Shall I spam this thread with pretty protein pics?
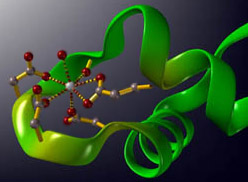
I have lots - dissertation on metalloproteins, and lots of lectures and papers! How about this one?Spoiler :
Calcium binding to the EF-hand domain of Calmodulin, for those of you who aren't as biochemistry-geeky as me.

MjM said:Im not on the front page?


 Now I get to use some pics from my dissertation! Amino acids can bind metal ions in these methods:
Now I get to use some pics from my dissertation! Amino acids can bind metal ions in these methods: 


