The Fjonis
Prince
Perfection - wow, multiple titrations! Never did that in chemistry... I have enough trouble finding out whether the bases / acids in such titrations are strong or weak..
Here are some graphs from a physics experiment where we let a pendulum oscillate under water at different depths. (oscillation number on x axis, amplitude on y axis. It turned out that the more submerged the pendulum bob was, the faster the bob slowed down (amplitude decreased faster). Enjoy!


Here are some graphs from a physics experiment where we let a pendulum oscillate under water at different depths. (oscillation number on x axis, amplitude on y axis. It turned out that the more submerged the pendulum bob was, the faster the bob slowed down (amplitude decreased faster). Enjoy!






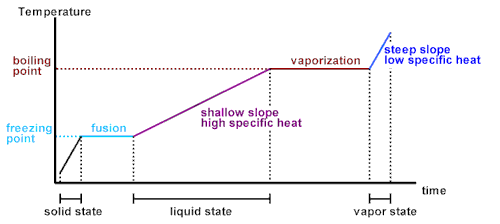
 ). I probably should've recognized something of it had I not skipped so many physics classes
). I probably should've recognized something of it had I not skipped so many physics classes  . I'm going to read into it more after work - thanks again for the clarification!
. I'm going to read into it more after work - thanks again for the clarification!

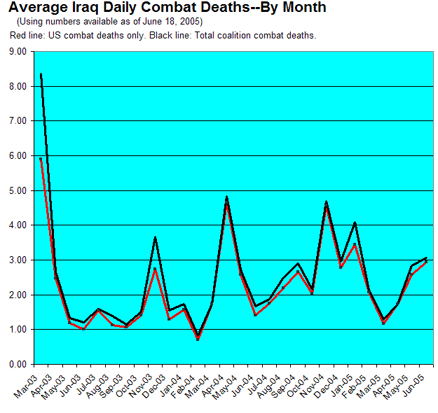



 (ok, fine, I'll speak for myself) :
(ok, fine, I'll speak for myself) :


