I think the bigger question is more how the interior is thermodynamically feasible, since there's no organelles, all chemistry is happening in the same big structure, how do the proteins and chemicals find each other, etc.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Newsworthy Science
- Thread starter Birdjaguar
- Start date
Broken_Erika
Play with me.
'She's perfect and she's beautiful': Frozen baby woolly mammoth discovered in Yukon gold fields
Whole baby mammoth, named Nun cho ga, is only 2nd found in the world, 1st in North America
https://www.cbc.ca/news/canada/north/frozen-whole-baby-woolly-mammoth-yukon-gold-fields-1.6501128
Whole baby mammoth, named Nun cho ga, is only 2nd found in the world, 1st in North America
https://www.cbc.ca/news/canada/north/frozen-whole-baby-woolly-mammoth-yukon-gold-fields-1.6501128
I think the bigger question is more how the interior is thermodynamically feasible, since there's no organelles, all chemistry is happening in the same big structure, how do the proteins and chemicals find each other, etc.
If I understand this correctly, these do have some sort of organelles
https://www.science.org/doi/10.1126/science.abb3634
These features, along with compartmentalization of genomic material and ribosomes in translationally active organelles bound by bioenergetic membranes, indicate gain of complexity in the Thiomargarita lineage and challenge traditional concepts of bacterial cells.
Using microwaves to drill geothermal boreholes to generate electricity
I love some of the Q+A's:

[EDIT] And then I came across this "advocacy" site, that is saying geothermal could be the answer to our electricity and heating needs
Quaise's ultra-deep geothermal power plan is one of the most exciting and fascinating green energy projects we've seen. In a nutshell, this Boston-based MIT spin-off says it has repurposed powerful millimeter-wave beam technology – originally developed to superheat plasma in fusion experiments – to blast through previously undrillable rock far below the Earth's surface.
"Geothermal plants only exist in places where natural conditions allow for energy extraction at relatively shallow depths of up to 400 feet (c 120m)," MIT said. Drill much deeper, and the heat of the Earth's crust wears drill bits out too quickly to be practical.
"If we can drill down to 20 kilometers, we can access these super-hot temperatures in greater than 90 percent of locations across the globe," Houde said.
By the end of 2022, Quaise wants to have vaporized a hole 10 times the depth of Woskov's lab experiments, and in 2023 it plans to vaporize a second hole 10 times deeper than the first. Houde said the planned depth of the second hole will give the company enough confidence to begin experiments in the field next year too. By 2026, Quaise wants to have an active pilot well that reaches temperatures of 500°C (932°F).
It'll be surprisingly quick, too. The deepest hole humanity has ever drilled to date took nearly 20 years to reach a depth of 12,289 m (40,318 ft), but Quaise says its hybrid drilling rig – using a traditional rotary bit to get through the easy stuff and a gyrotron-powered energy beam to melt, fracture and vaporize the tough stuff – will take just 100 days to deliver you a hole 20 km (12.4 miles) deep. Three and a bit months gets you a long-term green energy supply, in a stable bore hole lined with glassy melted rock, wherever you want it.
El Reg Q+A"Geothermal plants only exist in places where natural conditions allow for energy extraction at relatively shallow depths of up to 400 feet (c 120m)," MIT said. Drill much deeper, and the heat of the Earth's crust wears drill bits out too quickly to be practical.
"If we can drill down to 20 kilometers, we can access these super-hot temperatures in greater than 90 percent of locations across the globe," Houde said.
By the end of 2022, Quaise wants to have vaporized a hole 10 times the depth of Woskov's lab experiments, and in 2023 it plans to vaporize a second hole 10 times deeper than the first. Houde said the planned depth of the second hole will give the company enough confidence to begin experiments in the field next year too. By 2026, Quaise wants to have an active pilot well that reaches temperatures of 500°C (932°F).
It'll be surprisingly quick, too. The deepest hole humanity has ever drilled to date took nearly 20 years to reach a depth of 12,289 m (40,318 ft), but Quaise says its hybrid drilling rig – using a traditional rotary bit to get through the easy stuff and a gyrotron-powered energy beam to melt, fracture and vaporize the tough stuff – will take just 100 days to deliver you a hole 20 km (12.4 miles) deep. Three and a bit months gets you a long-term green energy supply, in a stable bore hole lined with glassy melted rock, wherever you want it.
I love some of the Q+A's:
Could this unleash the lizard people that inhabit the inner sphere?
How do we know they did not already drill up using this technology and are already amongst us?
Will this wake up the Kaiju?
No.
Isn't that how planet Krypton was destroyed?
No, an explosion destroyed Krypton.
How do we know they did not already drill up using this technology and are already amongst us?
Will this wake up the Kaiju?
No.
Isn't that how planet Krypton was destroyed?
No, an explosion destroyed Krypton.

[EDIT] And then I came across this "advocacy" site, that is saying geothermal could be the answer to our electricity and heating needs
After many years of failure to launch, new companies and technologies have brought geothermal out of its doldrums, to the point that it may finally be ready to scale up and become a major player in clean energy. In fact, if its more enthusiastic backers are correct, geothermal may hold the key to making 100 percent clean electricity available to everyone in the world. And as a bonus, it’s an opportunity for the struggling oil and gas industry to put its capital and skills to work on something that won’t degrade the planet.
Geothermal power, if it can be made to reliably and economically work in hotter, drier, and deeper rock, is a perfect complement to wind and solar. It is renewable and inexhaustible. It can run as baseload power around the clock, including at night, or “load follow” to complement renewables’ fluctuations. It is available almost everywhere in the world, a reliable source of domestic energy and jobs that, because it is largely underground, is resilient to most weather (and human) disasters. It can operate without pollution or greenhouse gases. The same source that makes the electricity can also be used to fuel district heating systems that decarbonize the building sector.
Geothermal power, if it can be made to reliably and economically work in hotter, drier, and deeper rock, is a perfect complement to wind and solar. It is renewable and inexhaustible. It can run as baseload power around the clock, including at night, or “load follow” to complement renewables’ fluctuations. It is available almost everywhere in the world, a reliable source of domestic energy and jobs that, because it is largely underground, is resilient to most weather (and human) disasters. It can operate without pollution or greenhouse gases. The same source that makes the electricity can also be used to fuel district heating systems that decarbonize the building sector.
Last edited:
tjs282
Stone \ Cold / Fish
I can see a chip attached to the metallic rings, so presumably the deformation to activate the telescopic sight is controlled via a smartphone-app or similar -- but I do wonder what powers it?
Narz
keeping it real
Pretty cool I dunno about mindblowing. 100x might blow my mind a lil
Ice Age wolf genomes home in on dog origins
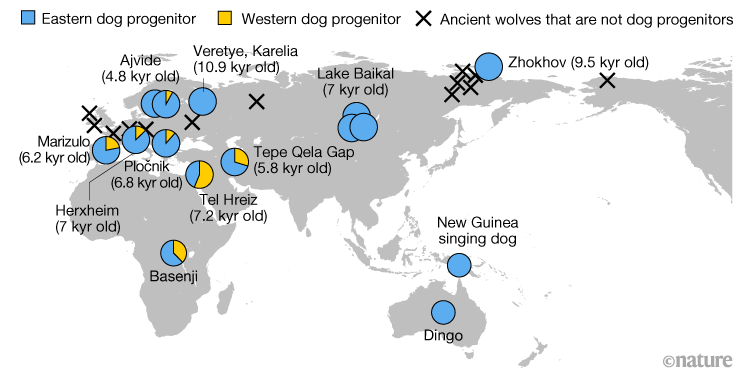
Paper Writeup
We charted the genetic history of the grey wolf over the past 100,000 years by analysing 72 ancient genomes. Placing dogs into this history, we found that they derive ancestry from at least two separate wolf populations.
I reckon I want a New Guinea singing dog
These dogs do not bark, and their chorused howling makes a haunting and extraordinary sound
They can also rotate their front and hind paws more than domestic dogs, which enables them to climb trees with thick bark or branches that can be reached from the ground; their climbing skills are closely related to those of a cat
Tim Flannery's report described them as "extraordinarily shy" and "almost preternaturally canny."
They can also rotate their front and hind paws more than domestic dogs, which enables them to climb trees with thick bark or branches that can be reached from the ground; their climbing skills are closely related to those of a cat
Tim Flannery's report described them as "extraordinarily shy" and "almost preternaturally canny."

Paper Writeup
You know that Daredevil echolocation thing? People can really do that!
Human click-based echolocation: Effects of blindness and age, and real-life implications in a 10-week training program

The average proportion of successful maze completions in sessions 1–20.
In session 15, unpredictable starting orientations were introduced, along with a 15 s timeout when a collision occurred. This is represented by the dashed black line. Data from SCs and BCs are shown as black and white circles respectively, with each symbol representing the average and error bars representing the standard error of the mean across participants. Data from experts (n = 4) who completed only a single session without training are shown as grey circles. For comparison, they have been plotted at session 14.
Human click-based echolocation: Effects of blindness and age, and real-life implications in a 10-week training program
We report a training study investigating the effects of blindness and age on the learning of a complex auditory skill: click-based echolocation. Blind and sighted participants of various ages (21–79 yrs; median blind: 45 yrs; median sighted: 26 yrs) trained in 20 sessions over the course of 10 weeks in various practical and virtual navigation tasks. Blind participants also took part in a 3-month follow up survey assessing the effects of the training on their daily life.
We found that both sighted and blind people improved considerably on all measures, and in some cases performed comparatively to expert echolocators at the end of training.
Furthermore, in the follow up survey, all participants who were blind reported improved mobility, and 83% reported better independence and wellbeing. Overall, our results suggest that the ability to learn click-based echolocation is not strongly limited by age or level of vision. This has positive implications for the rehabilitation of people with vision loss or in the early stages of progressive vision loss.
We found that both sighted and blind people improved considerably on all measures, and in some cases performed comparatively to expert echolocators at the end of training.
Furthermore, in the follow up survey, all participants who were blind reported improved mobility, and 83% reported better independence and wellbeing. Overall, our results suggest that the ability to learn click-based echolocation is not strongly limited by age or level of vision. This has positive implications for the rehabilitation of people with vision loss or in the early stages of progressive vision loss.
The average proportion of successful maze completions in sessions 1–20.
In session 15, unpredictable starting orientations were introduced, along with a 15 s timeout when a collision occurred. This is represented by the dashed black line. Data from SCs and BCs are shown as black and white circles respectively, with each symbol representing the average and error bars representing the standard error of the mean across participants. Data from experts (n = 4) who completed only a single session without training are shown as grey circles. For comparison, they have been plotted at session 14.
I assumed that it is not about the benefit to sighted people, but the general principle that you should examine how widely applicable your results are. If they only had tested blind people and reported that, people may assume it is a feature of blindness. This shows that anyone can learn it.What is the benefit for sighted people?
Broken_Erika
Play with me.
Scientists discover a new species of giant water lily that can grow up to 3 metres wide
The Victoria boliviana also has sharp spikes around their rims, says researcher Natalia Przelomska
Researchers in London say they have identified a new species of the giant water lily plant — after discovering it had been sitting serenely in their collection for 177 years.
The new species, dubbed Victoria boliviana, was at first thought to be one of the two previously known species, Victoria amazonica and Victoria cruziana, because it was so similar.
The overall genus Victoria was originally named after the U.K.'s Queen Victoria; boliviana comes from the fact that it originated from Bolivia.
But a closer look reveals several unique characteristics.
"They have these huge floating round leaves," Natalia Przelomska, a biodiversity genomics researcher with the Royal Botanic Garden, Kew, told As It Happens guest host Ginella Massa. "The leaves can grow to over 10 feet in diameter," or more than three metres.
https://www.cbc.ca/radio/asithappen...y-that-can-grow-up-to-3-metres-wide-1.6511116
The Victoria boliviana also has sharp spikes around their rims, says researcher Natalia Przelomska
Researchers in London say they have identified a new species of the giant water lily plant — after discovering it had been sitting serenely in their collection for 177 years.
The new species, dubbed Victoria boliviana, was at first thought to be one of the two previously known species, Victoria amazonica and Victoria cruziana, because it was so similar.
The overall genus Victoria was originally named after the U.K.'s Queen Victoria; boliviana comes from the fact that it originated from Bolivia.
But a closer look reveals several unique characteristics.
"They have these huge floating round leaves," Natalia Przelomska, a biodiversity genomics researcher with the Royal Botanic Garden, Kew, told As It Happens guest host Ginella Massa. "The leaves can grow to over 10 feet in diameter," or more than three metres.
https://www.cbc.ca/radio/asithappen...y-that-can-grow-up-to-3-metres-wide-1.6511116
There is a mindfullness study in the news. The beeb reports it as:


Executive Function (p=7%)

Social Behaviour (p=8%)

Mindfulness outcome (negative effect size, p=99%)

School mindfulness lessons don't work for teenagers, study says
Giving teenagers mindfulness lessons at school to boost wellbeing is largely a waste of time, a major UK study has found.
The technique, which encourages people to meditate and live in the moment, was no better than what schools were already doing for mental health.
However the study, a meta analysis of 66 randomised controlled trials, say this:Giving teenagers mindfulness lessons at school to boost wellbeing is largely a waste of time, a major UK study has found.
The technique, which encourages people to meditate and live in the moment, was no better than what schools were already doing for mental health.
Sixty-six RCTs, involving 20,138 participants (9,552 receiving an MBP and 10,586 controls), were identified. Compared with passive controls, MBPs were effective in improving anxiety/stress, attention, executive functioning, and negative and social behaviour (d from 0.12 to 0.35). Compared against active controls, MBPs were more effective in reducing anxiety/stress and improving mindfulness (d=0.11 and 0.24, respectively). In studies with a follow-up, there were no significant positive effects of MBPs. No consistent pattern favoured MBPs as a universal versus selective intervention.
The points that I would raise with the beeb interpretation:
- Absence of evidence is not evidence of absence. Sure, none of the effects at followup reached significance, but all the effect sizes are in the same direction, except the -0.01 for "Mindfullness". A couple of the p values were close to 5%, but to be honest there is an argument that they have a uniform distribution (table 2 below, P vale of Intervention effects).
- There is obviously something going on. Heterogeneity P value is significant for all effects except well-being. I would interpret this as mindfulness programs have a vast difference in effectiveness, rather than none of them work.
- They are all compared to "selective intervention", rather than the doing nothing that is the usual experience for children in the UK with such problems. This is not pointed out by the beeb at all.
- They work at "reducing anxiety/stress and improving mindfulness". Is that not of some value?
Spoiler Results tables and forest plots :


Executive Function (p=7%)

Social Behaviour (p=8%)

Mindfulness outcome (negative effect size, p=99%)

Last edited:
I feel I should preface this post with: Do not stop taking any medication perscribed by your doctor. This is interesting but early and controversial work that may or may not translate to treatment decisions some time down the road.
No link between depression and serotonin, finds major analysis
Writeup Paper
No link between depression and serotonin, finds major analysis
There may be no link between serotonin levels and depression, according to an analysis of 17 studies. This raises questions about antidepressants that focus on this brain-signalling molecule, say the authors of the analysis. Not everyone is convinced by the findings, though.
The serotonin hypothesis, which dates from the 1960s, says that a chemical imbalance in the brain, including low levels of serotonin, also known as 5-hydroxytryptamine or 5-HT, leads to depression. We now think various biological, psychological and environmental factors play a role, but the most popular antidepressants, known as selective serotonin reuptake inhibitors (SSRIs), increase the availability of serotonin in the brain.
Now, Joanna Moncrieff at University College London and her colleagues have done an “umbrella analysis” of 17 systematic reviews and studies, which together included hundreds of thousands of people with and without depression.
It is difficult to directly measure real-time serotonin levels in the brain, so the 17 studies looked at depression and proxies for serotonin, such as the molecules in cerebral fluid that serotonin breaks down into; the levels of serotonin receptors and how active they are; or whether there are more genes for serotonin transporters – which remove serotonin – in people with depression.
Moncrieff’s team found that there was no evidence that low serotonin activity or amounts cause depression.
“The implication of our paper is that we do not know what [SSRI] antidepressants are doing,” says Moncrieff. One possibility is that they are working through a placebo effect, she says.
However, Johan Lundberg at the Karolinska Institute in Sweden says a limitation of the analysis is that it didn’t distinguish between people who had ongoing depression and those who have episodes of depression, whose state at the time they were assessed could affect the functioning of their serotonin systems. “It is key to separately analyse data from studies that examine the same patients when ill and when in remission, to have optimal conditions to examine the hypothesis,” he says.
“Antidepressants are an effective, NICE-recommended treatment for depression that can also be prescribed for a range of physical and mental health conditions,” a spokesperson for the Royal College of Psychiatrists told the Science Media Centre in the UK, referring to treatment guidelines from the National Institute for Health and Care Excellence (NICE) in England. “Antidepressants will vary in effectiveness for different people, and the reasons for this are complex. We would not recommend for anyone to stop taking their antidepressants based on this review, and encourage anyone with concerns about their medication to contact their [family doctor].”
The serotonin hypothesis, which dates from the 1960s, says that a chemical imbalance in the brain, including low levels of serotonin, also known as 5-hydroxytryptamine or 5-HT, leads to depression. We now think various biological, psychological and environmental factors play a role, but the most popular antidepressants, known as selective serotonin reuptake inhibitors (SSRIs), increase the availability of serotonin in the brain.
Now, Joanna Moncrieff at University College London and her colleagues have done an “umbrella analysis” of 17 systematic reviews and studies, which together included hundreds of thousands of people with and without depression.
It is difficult to directly measure real-time serotonin levels in the brain, so the 17 studies looked at depression and proxies for serotonin, such as the molecules in cerebral fluid that serotonin breaks down into; the levels of serotonin receptors and how active they are; or whether there are more genes for serotonin transporters – which remove serotonin – in people with depression.
Moncrieff’s team found that there was no evidence that low serotonin activity or amounts cause depression.
“The implication of our paper is that we do not know what [SSRI] antidepressants are doing,” says Moncrieff. One possibility is that they are working through a placebo effect, she says.
However, Johan Lundberg at the Karolinska Institute in Sweden says a limitation of the analysis is that it didn’t distinguish between people who had ongoing depression and those who have episodes of depression, whose state at the time they were assessed could affect the functioning of their serotonin systems. “It is key to separately analyse data from studies that examine the same patients when ill and when in remission, to have optimal conditions to examine the hypothesis,” he says.
“Antidepressants are an effective, NICE-recommended treatment for depression that can also be prescribed for a range of physical and mental health conditions,” a spokesperson for the Royal College of Psychiatrists told the Science Media Centre in the UK, referring to treatment guidelines from the National Institute for Health and Care Excellence (NICE) in England. “Antidepressants will vary in effectiveness for different people, and the reasons for this are complex. We would not recommend for anyone to stop taking their antidepressants based on this review, and encourage anyone with concerns about their medication to contact their [family doctor].”
Writeup Paper
Well, if it is a placebo effect, it is pretty effective. Maybe the solution is to multiply self-suggestion until you are perfectly happyI feel I should preface this post with: Do not stop taking any medication perscribed by your doctor. This is interesting but early and controversial work that may or may not translate to treatment decisions some time down the road.
No link between depression and serotonin, finds major analysis
There may be no link between serotonin levels and depression, according to an analysis of 17 studies. This raises questions about antidepressants that focus on this brain-signalling molecule, say the authors of the analysis. Not everyone is convinced by the findings, though.The serotonin hypothesis, which dates from the 1960s, says that a chemical imbalance in the brain, including low levels of serotonin, also known as 5-hydroxytryptamine or 5-HT, leads to depression. We now think various biological, psychological and environmental factors play a role, but the most popular antidepressants, known as selective serotonin reuptake inhibitors (SSRIs), increase the availability of serotonin in the brain.Now, Joanna Moncrieff at University College London and her colleagues have done an “umbrella analysis” of 17 systematic reviews and studies, which together included hundreds of thousands of people with and without depression.It is difficult to directly measure real-time serotonin levels in the brain, so the 17 studies looked at depression and proxies for serotonin, such as the molecules in cerebral fluid that serotonin breaks down into; the levels of serotonin receptors and how active they are; or whether there are more genes for serotonin transporters – which remove serotonin – in people with depression.Moncrieff’s team found that there was no evidence that low serotonin activity or amounts cause depression.“The implication of our paper is that we do not know what [SSRI] antidepressants are doing,” says Moncrieff. One possibility is that they are working through a placebo effect, she says.However, Johan Lundberg at the Karolinska Institute in Sweden says a limitation of the analysis is that it didn’t distinguish between people who had ongoing depression and those who have episodes of depression, whose state at the time they were assessed could affect the functioning of their serotonin systems. “It is key to separately analyse data from studies that examine the same patients when ill and when in remission, to have optimal conditions to examine the hypothesis,” he says.“Antidepressants are an effective, NICE-recommended treatment for depression that can also be prescribed for a range of physical and mental health conditions,” a spokesperson for the Royal College of Psychiatrists told the Science Media Centre in the UK, referring to treatment guidelines from the National Institute for Health and Care Excellence (NICE) in England. “Antidepressants will vary in effectiveness for different people, and the reasons for this are complex. We would not recommend for anyone to stop taking their antidepressants based on this review, and encourage anyone with concerns about their medication to contact their [family doctor].”
Writeup Paper

EgonSpengler
Deity
- Joined
- Jun 26, 2014
- Messages
- 12,260
A woman on the radio cited a study that estimated the economic costs of gun violence in the United States at $557,000,000,000 per year. She said that's a conservative estimate, because it only considered the measurable costs of things like emergency services, police investigations, medical care and lost income & wages. It didn't even try to put an economic cost to other 'knock-on' effects, such as the 87% increase in substance abuse disorders among people who survive and recover from gunshot wounds, or the 12% increase in psychiatric disorders among the family members of people who are shot. Sorry I don't have a link yet. I'd like to go look up the study. This is precisely the kind of thing the violence enthusiasts were afraid of when they actively prevented studies of the impact of gun violence for all those years. They better put on a helmet. I suspect this is only the beginning of the avalanche of bad news coming down the mountain.
Reaching out to old acquaintances (perhaps after a covid related absence) is more appreciated than you think


Peggy Liu at the University of Pittsburgh and her colleagues have been using questionnaires and field experiments among college students to see how well people respond to being contacted by old acquaintances. Those doing the reaching out consistently underestimated how graciously and positively their messages would be received. We are also quite useless at asking each other for favours, says the study. We worry about what the favour will cost our friends, while forgetting how rotten they would feel if they refused to help.
Abstact
People are fundamentally social beings and enjoy connecting with others. Sometimes, people reach out to others—whether simply to check-in on how others are doing with brief messages or to show that they are thinking of others by sending small gifts to them. Yet, despite the importance and enjoyment of social connection, do people accurately understand how much other people value being reached out to by someone in their social circle? Across a series of preregistered experiments, we document a robust underestimation of how much other people appreciate being reached out to. We find evidence compatible with an account wherein one reason this underestimation of appreciation occurs is because responders (vs. initiators) are more focused on their feelings of surprise at being reached out to. A focus on feelings of surprise in turn predicts greater appreciation. We further identify process-consistent moderators of the underestimation of reach-out appreciation, finding that it is magnified when the reach-out context is more surprising: when it occurs within a surprising (vs. unsurprising) context for the recipient and when it occurs between more socially distant (vs. socially close) others. Altogether, this research thus identifies when and why we underestimate how much other people appreciate us reaching out to them, implicating a heightened focus on feelings of surprise as one underlying explanation.


Last edited:
THE WEEKEND INTERVIEW with Kevin Tracey
| By Allysia Finley
Electricity Is the New Medical Miracle
Kelly Owens was a medical mystery, her teens and 20s blighted by a cascade of seemingly unrelated health problems that left her debilitated. For a decade and a half she was put on one medication after another— 22 in all—to little effect. Then electricity saved her. “I didn’t even remember how ‘ healthy’ felt, since it had been 15 years,” Ms. Owens, 33, says. Now she and her husband are talking about having a child, something she had thought impossible. She credits Kevin Tracey, an innovative neurosurgeon she found through Facebook.
Ms. Owens was an athletic 13-year-old when she twisted her ankle tap-dancing. A few weeks later, her ankle was still swollen and she began experiencing severe nausea and diarrhea. A year or two later, her other ankle swelled up, though she’d never injured it. Then her knees grew inflamed. After a colonoscopy and endoscopy, she was diagnosed with Crohn’s disease, an inflammatory bowel condition. Blood tests and a physical examination revealed spondyloarthropathy arthritis, which attacked her spine, joints and organs. She developed blood clots and skin ulcers. By the time she finished college, she says, there wasn’t a joint in her body that didn’t hurt. Her myriad ailments made it difficult to walk and forced her to quit her job as a teacher. To control her joint inflammation, she was prescribed steroids, which made her bones as brittle as a 70-year-old woman’s. She was 25 when she stumbled on a Facebook video of Dr. Tracey, CEO of the Manhasset, N.Y.-based Feinstein Institutes for Medical Research, discussing how electricity could replace medication.
Dr. Tracey, 64, pioneered research showing that electrical stimulation of the vagus nerve—the nervous- system “motherboard” that originates at the back of the neck, which connects the brain to the rest of the body—could suppress inflammation that causes chronic diseases such as Crohn’s and rheumatoid arthritis.
| By Allysia Finley
Electricity Is the New Medical Miracle
Kelly Owens was a medical mystery, her teens and 20s blighted by a cascade of seemingly unrelated health problems that left her debilitated. For a decade and a half she was put on one medication after another— 22 in all—to little effect. Then electricity saved her. “I didn’t even remember how ‘ healthy’ felt, since it had been 15 years,” Ms. Owens, 33, says. Now she and her husband are talking about having a child, something she had thought impossible. She credits Kevin Tracey, an innovative neurosurgeon she found through Facebook.
Ms. Owens was an athletic 13-year-old when she twisted her ankle tap-dancing. A few weeks later, her ankle was still swollen and she began experiencing severe nausea and diarrhea. A year or two later, her other ankle swelled up, though she’d never injured it. Then her knees grew inflamed. After a colonoscopy and endoscopy, she was diagnosed with Crohn’s disease, an inflammatory bowel condition. Blood tests and a physical examination revealed spondyloarthropathy arthritis, which attacked her spine, joints and organs. She developed blood clots and skin ulcers. By the time she finished college, she says, there wasn’t a joint in her body that didn’t hurt. Her myriad ailments made it difficult to walk and forced her to quit her job as a teacher. To control her joint inflammation, she was prescribed steroids, which made her bones as brittle as a 70-year-old woman’s. She was 25 when she stumbled on a Facebook video of Dr. Tracey, CEO of the Manhasset, N.Y.-based Feinstein Institutes for Medical Research, discussing how electricity could replace medication.
Dr. Tracey, 64, pioneered research showing that electrical stimulation of the vagus nerve—the nervous- system “motherboard” that originates at the back of the neck, which connects the brain to the rest of the body—could suppress inflammation that causes chronic diseases such as Crohn’s and rheumatoid arthritis.
She enrolled in a vagus-nerve stimulation trial by SetPoint Medical, a California-based biotech startup that Dr. Tracey co-founded in 2007. With financial help from family and friends, she and her husband moved to Amsterdam, one of the sites where the trial took place. The trial’s principal investigator was Geert D’Haens, a global expert in inflammatory bowel disease based at the Amsterdam University Medical Center. SetPoint implanted a pacemaker- sized device in her chest cavity that sends stimulation to electrodes surgically placed on her vagus nerve. Her symptoms began to improve within weeks. Soon she was able not only to walk but to run. Two months after the device was implanted, doctors deemed her in clinical remission. Her ailments have remained at bay, and her doctors weaned her from steroids.
Scientists have long known that the vagus nerve carries signals between the brain and internal organs that regulate physiological processes such as digestion, breathing and heart rate. When you exercise, for instance, your heart speeds up. Then your brain sends a signal via the vagus nerve directing your heart to slow down so it doesn’t beat out of control. Dr. Tracey’s breakthrough two decades ago was the discovery that the brain also controls the immune system through the vagus nerve. By using electrical stimulation to hack into neural networks, it’s possible to regulate the immune response and perhaps someday cure inflammatory conditions such as multiple sclerosis, lupus and even Alzheimer’s disease.
The story of this novel insight begins with Dr. Tracey’s painful childhood. His mother died of an inoperable brain tumor when he was 5. That sparked his interest in neurosurgery. He wanted to develop treatments so that other children wouldn’t have to suffer the way he and his two younger siblings had. He went to medical school and joined a New York hospital as a neurosurgery resident.
In 1985 he was caring for an 11-month-old girl named Janice. “She’d been crawling across the kitchen floor when her grandmother was cooking dinner. And grandma turned to drain boiling water in the sink and spilled the boiling water on her granddaughter,” Dr. Tracey recalls. “We didn’t think she was going to survive. But she did—she survived for a month—and then inexplicably went into shock and died in my arms. And so I was haunted by her death. She died of septic shock.”
Septic shock occurs when a nonfatal injury or infection leads to organ failure and dangerously low blood pressure. Sepsis causes 1 in 5 deaths worldwide. In 1985 scientists didn’t understand what causes the condition. Janice’s death spurred Dr. Tracey to research sepsis’ biological underpinnings: “What we discovered is that the molecule that killed Janice was made by her own immune system. It’s a molecule that’s known today as TNF”—tumor necrosis factor. TNF is a cytokine, a protein made by the immune system to send signals that can cause or reduce inflammation. But the discovery of TNF explained only part of the mystery behind sepsis. Questions remained, Dr. Tracey said: “What is it that controls the amount of cytokines being produced? Why do some people, like Janice, make massive amounts of cytokines that can kill them?”
While testing an experimental drug that blocked TNF production in mice with strokes, his lab stumbled on a clue. The drug not only blocked TNF production in the rodents’ brains, which helped the strokes heal; it also turned off TNF and other cytokines made in the rest of the body. That led to the discovery that “the brain communicates to these organs by sending signals through the vagus nerve.” His lab performed two more experiments in mice that confirmed this hypothesis. “So now we knew the vagus nerve could transmit this off-switch to the immune system.” He postulated that “if there’s an off-switch in the vagus nerve, there must be an on-switch, which is how a reflex works.” More experimentation and research proved his hunch true.
In the case of sepsis, bacteria activate white blood cells to produce cytokines, which can help heal wounds. An inflammatory condition like Ms. Owens’s can also trigger the release of cytokines. Problems arise when the nervous system fails to regulate the production of cytokines. “If the nervous system doesn’t control that response, the immune system can overproduce cytokines,” which can result in autoimmune conditions like rheumatoid arthritis and Crohn’s disease, Dr. Tracey says. Hence the treatment: “You can implant a device on the vagus nerve of humans, or animals, and by controlling the activity of the nerve with the nerve stimulating device, you can control the magnitude of the cytokine response.”
The vagus nerve is actually a network of some 160,000 nerve fibers, 80,000 on either side of the neck. Each fiber has a specific job—for instance, controlling heart rate. These fibers also deliver information to the brain, which processes them and sends signals back down the vagus nerve or to nearby structures such as the pituitary gland, which regulates hormone production.
Stimulating the vagus nerve can relieve arthritis, Crohn’s disease and other inflammatory conditions— perhaps someday even Alzheimer’s disease.


BARBARA KELLEY
How do doctors know which fiber or fibers to stimulate? Dr. Tracey explains a “ cool trick called optogenetics,” which involves genetically engineering mice so that the fibers in their brain stem are stimulated to send signals to the body when activated by a laser beam. Researchers can then figure out which fibers control which processes by shining a laser on the neurons. More than 100 trials world- wide are being conducted using vagus-nerve stimulation for an array of conditions. SetPoint has conducted three small trials on vagus-nerve stimulation for rheumatoid arthritis and Crohn’s. “The same device implanted in the same location can be used for other diseases,” says CEO Murthy Simhambhatla. (Dr. Tracey resigned from the company’s board in 2011 to spend more time in his lab after meeting the first rheumatoid-arthritis patient treated in a SetPoint clinical trial who experienced complete remission. He continues to work as a consultant for SetPoint.)
Eight of the 16 patients in Ms. Owens’s trial showed improvement after four months, and she and three others went into complete remission. SetPoint plans to conduct larger randomized controlled trials on patients who haven’t responded to biologic drugs. Such trials can take many years to complete, as they do for drugs, but the Food and Drug Administration has been helpful in supporting the innovation. Last year the FDA approved the technique to help people who have suffered damage to motor skills caused by strokes.
Vagus-nerve stimulation might also help some people suffering from “ long Covid,” Dr. Tracey says, although he cautions more research is needed. A study earlier this year found that most long-Covid patients had signs pointing to vagus-nerve dysfunction, including diarrhea, dizziness and rapid heart rate. Many also showed signs of vagus-nerve damage on medical imaging. Some patients may shudder at the idea of getting implanted with a device that sends electrical pulses up to their brains and back down to the body. “Some have been quick to say, well, vagus-nerve stimulation is invasive,” Dr. Tracey says. “Well, I would say that biologics are invasive too. They’re administered with needles.” He adds that the 150,000 or so epilepsy patients who have been treated with vagus-nerve stimulation over the decades have very rarely experienced side effects. Some drugs also work by chemically stimulating the vagus nerve and may carry potential to treat conditions other than those for which they were originally developed. Dr. Tracey conducted a small trial that found famotidine (also known by the brand name Pepcid) can reduce the duration of acute Covid in patients with mild to moderate symptoms by activating the vagus nerve and suppressing the cytokine storm. Healthy behaviors like exercise and meditation can also stimulate the vagus nerve, Dr. Tracey says, but they may not help patients whose nerve fibers are damaged or who have a genetic predisposition. The latter might have caused Ms. Owens’s ailments.
Dr. Tracey is reluctant to say she’s cured: “She might be. We don’t know. How do you know if she’s cured? No one wants to turn the device off.” But she feels like a normal, healthy 33-year-old, and she hopes her story will inspire others with similar conditions: “Patients really need to have hope.”
Ms. Finley is a member of the Journal’s editorial board.
Hepatitis cases in children linked to adeno-associated virus AAV2 (press summary, I think this is one paper and I cannot find the other paper)
There was a worrying spike in acute hepatitis cases in children all around the world. A couple of UK teams have produced pretty good evidence it is caused by co-infection of two relatively benign visuses. It is likely that the spike of infections accosiated with coming out of lockdown was the cause.
There was a worrying spike in acute hepatitis cases in children all around the world. A couple of UK teams have produced pretty good evidence it is caused by co-infection of two relatively benign visuses. It is likely that the spike of infections accosiated with coming out of lockdown was the cause.
Since April 2022, a number of young children worldwide have developed jaundice and acute severe hepatitis of unknown origin. Currently the World Health Organisation (WHO) has reported at least 1010 probable cases in 35 countries. Children with the condition have commonly had to be hospitalised for a number of days, with 11 children in England and one in Scotland requiring a liver transplant. In the UK, the majority of the 268 cases have been under the age of five years old with nearly 40% of hospitalised cases (74 of 189) requiring admission to intensive care.
The two studies were led independently and simultaneously. One, examining cases from Scotland by the MRC-University of Glasgow Centre for Virus Research (CVR) and the Royal Hospital for Children in Glasgow, in partnership with Public Health Scotland and ISARIC (International Severe Acute Respiratory and emerging Infections Consortium) WHO Clinical Characterisation Protocol UK (CCP-UK); and a second studying cases from across all four UK nations at Great Ormond Street Hospital and the UCL Great Ormond Street Institute of Child Health (UCL GOS ICH), in partnership with the UK Health Security Agency.
Previously, health officials had thought that a spike in adenovirus infections in spring 2022 – until now the most commonly-found virus in samples from the affected children – may be part of the explanation for this spike in hepatitis cases. These two new studies shed light on another virus that seems to play a significant role.
Researchers across two teams found that AAV2 (which cannot replicate without a ‘helper’ virus such as an adenovirus or herpesvirus) was present in 96% cases of unknown hepatitis examined across both studies.
Therefore, the researchers believe that coinfection with two viruses – AAV2 and an adenovirus, or less often the herpes virus HHV6 (which has also been found in sample from some patients) – may offer the best explanation for the onset of severe liver disease in affected children.
The Scottish study carried out a detailed investigation of nine cases and 58 control subjects. Using next-generation sequencing and real-time PCR, the research team compared samples and were able to confirm the presence of AAV2 in the plasma and liver of all nine cases. There was no AAV2 in any of the subjects in the control groups, which were made up of age-matched healthy controls, children with adenovirus but normal liver function and children admitted to hospital with known causes of hepatitis.
With expertise in metagenomics and adenovirus sequencing, the London team studied 28 cases, including liver samples from five children that required a transplant and blood samples from the remaining children who did not – residual samples were sufficient to test 17 cases for AAV2, 16 of which tested positive. RNA sequencing of liver samples confirmed the presence of AAV2 replication in the liver of children with unknown hepatitis.
Last edited:
