You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
[RD] Daily Graphs and Charts II: Another 10,000 to come.
- Thread starter Cutlass
- Start date
They are some data company paid by people like the World Wildlife Fund to make reports like this.Who are these Ember people?
danjuno
Cole Phelps, Badge 1247
Thorgalaeg
Deity
Would be more interesting with tangential speeds. So, the angular speed showed in that animation multiplied by radius. That would show how crazy Jupiter is.
I want to do an experiment remaking this chart with glasses of these extracts as rows and household acids/bases as columns.


^^^^ I do not understand the colors.
Well, exactly what they will look like in the real world I do not know, but if I do it I shall take a picture of it and post it here. If tomato goes completely white when exposed to strong acid I shall be amazed. You get the basic idea of a pH indicator, and have seen the colour change in red cabbage? This has a writeup of the science.^^^^ I do not understand the colors.
Spoiler Using Food as Natural Indicators :
We can classify different solutions depending on their acidic and basic properties. We
define a pH scale to do this, ranging from value of well below 0 to 14 or higher. Solutions are acidic if they have
a pH below 7, and basic if their pH is above 7. pH 7 is a neutral solution.
Indicators are really useful tools to tell whether a solution we have is acidic or basic. They
have excellent properties in which they change colour depending on the pH of the solution
they are in. They achieve this by subtle changes to the molecule that gives rise to their
colour when exposed to the different acidic/basic environments.
Red cabbage and turmeric [labelled above as curry powder] are just two examples many useful natural indicators.
Turmeric displays two colours depending on whether it is exposed to an acidic or basic
solution. The molecule responsible for the colourful properties of turmeric is curcumin:

The subtle change in the structure of the molecule gives rise to the colour change from
yellow to red when in acidic and basic solutions. Therefore, turmeric lends itself as a really
useful, natural indicator!
Red cabbage indicator is even more useful, as it displays a range of colours which can be
compared to a colour chart to determine not only if the solution is acidic or basic, but the
rough pH of the solution.
define a pH scale to do this, ranging from value of well below 0 to 14 or higher. Solutions are acidic if they have
a pH below 7, and basic if their pH is above 7. pH 7 is a neutral solution.
Indicators are really useful tools to tell whether a solution we have is acidic or basic. They
have excellent properties in which they change colour depending on the pH of the solution
they are in. They achieve this by subtle changes to the molecule that gives rise to their
colour when exposed to the different acidic/basic environments.
Red cabbage and turmeric [labelled above as curry powder] are just two examples many useful natural indicators.
Turmeric displays two colours depending on whether it is exposed to an acidic or basic
solution. The molecule responsible for the colourful properties of turmeric is curcumin:
The subtle change in the structure of the molecule gives rise to the colour change from
yellow to red when in acidic and basic solutions. Therefore, turmeric lends itself as a really
useful, natural indicator!
Red cabbage indicator is even more useful, as it displays a range of colours which can be
compared to a colour chart to determine not only if the solution is acidic or basic, but the
rough pH of the solution.
Last edited:
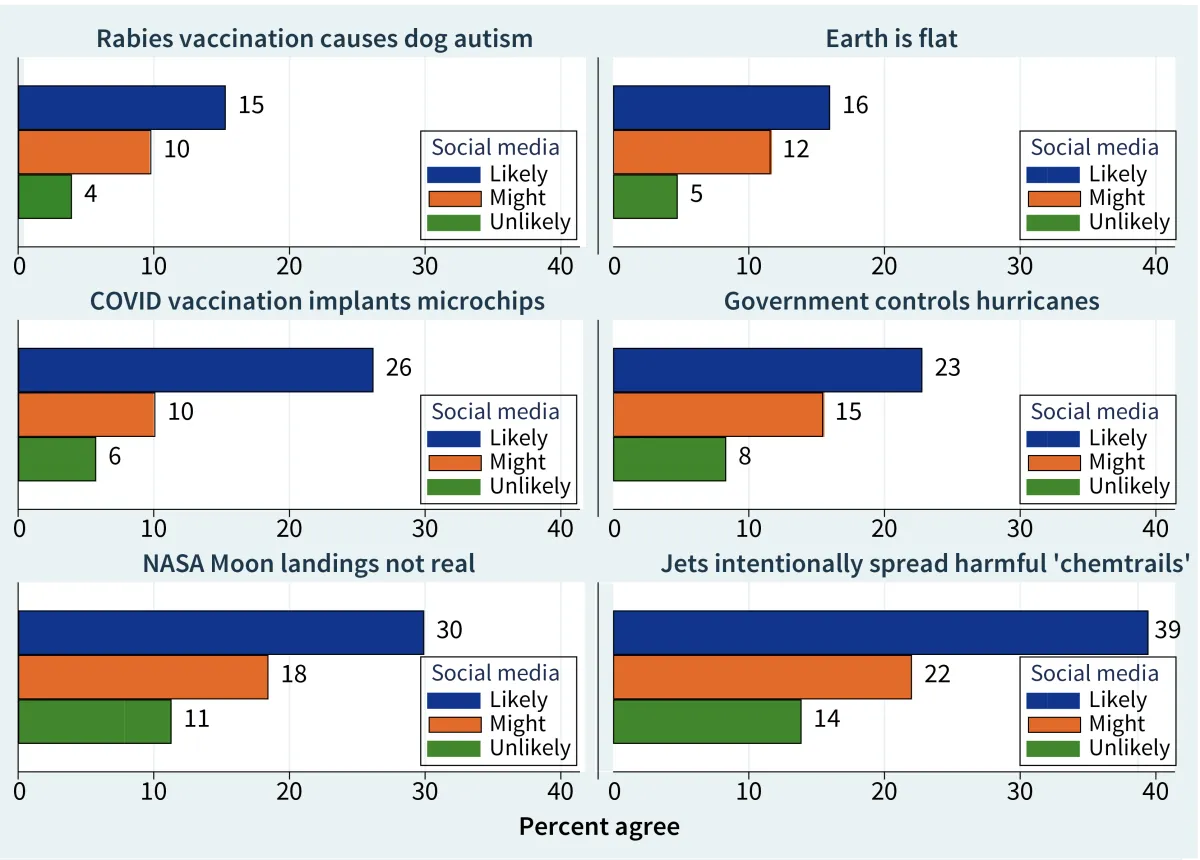
Can the Government Control Hurricanes? New Survey Results on Conspiracies and Science


Spoiler More from paper :
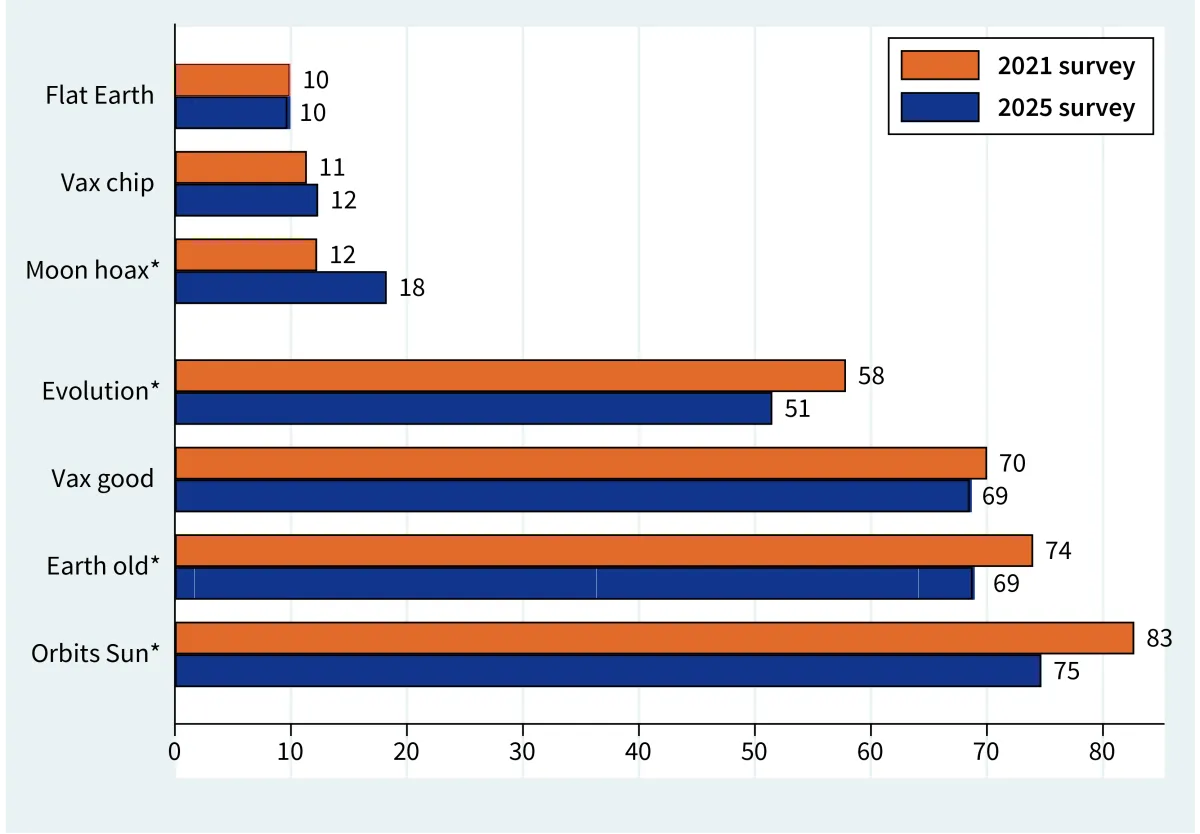
Percent agreement with three conspiracy and four science items that were carried on both 2021 and 2025 surveys
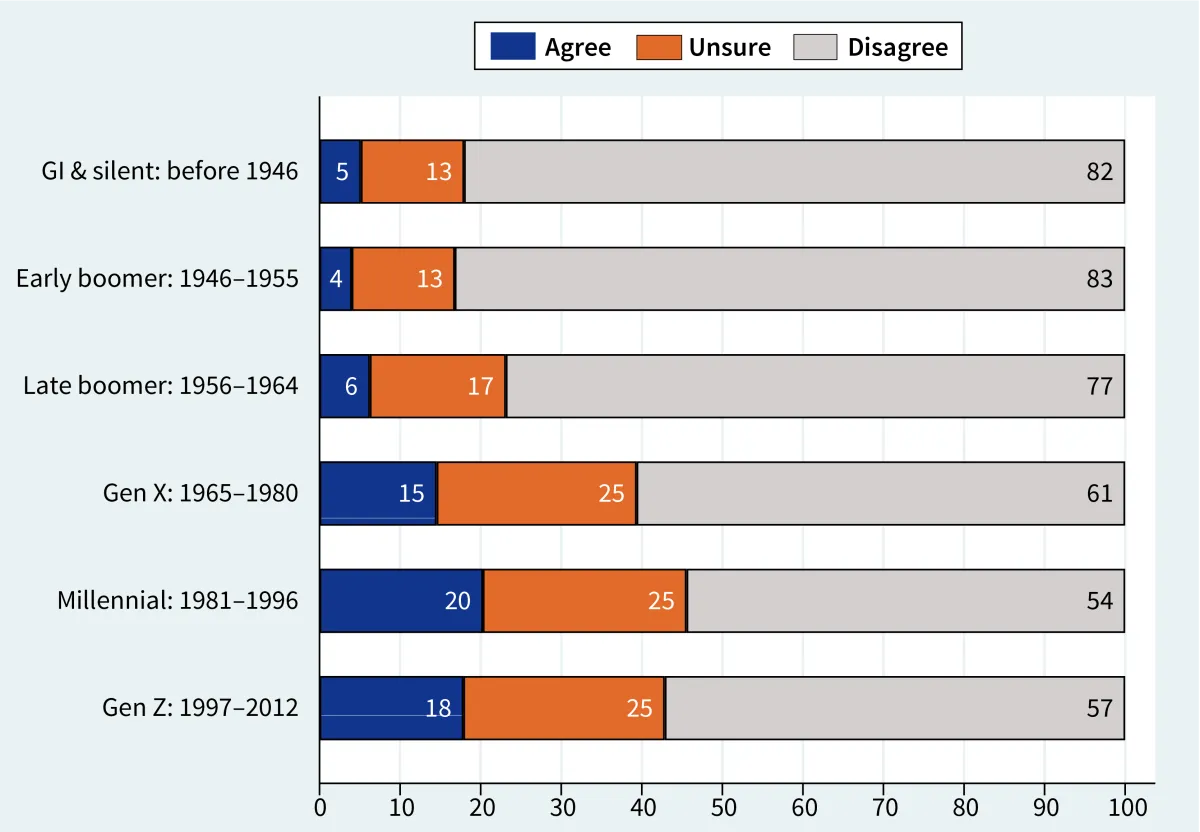
 Percent agree/disagree that government has the ability to control hurricanes, by respondent’s generation.
Percent agree/disagree that government has the ability to control hurricanes, by respondent’s generation.
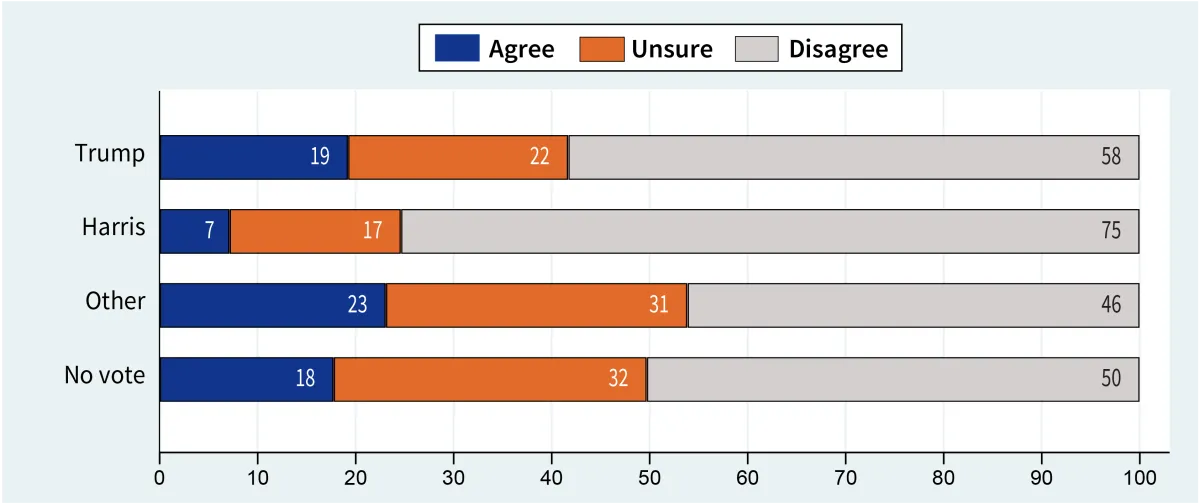
 Percent agree/disagree that government has the ability to control hurricanes, by vote in 2024 presidential election
Percent agree/disagree that government has the ability to control hurricanes, by vote in 2024 presidential election
 Percent agree with six conspiracies, by how likely respondent is to check social media for information about science-related topics.
Percent agree with six conspiracies, by how likely respondent is to check social media for information about science-related topics.




Similar threads
pre-release info
Detailed Civ and Leader Comparison Charts
- Replies
- 55
- Views
- 14K
- Replies
- 4
- Views
- 457
- Replies
- 128
- Views
- 14K
- Replies
- 350
- Views
- 22K